Plugged In: A Lasagna-Lover’s Guide to EV Battery Cell Anatomy

Last Updated on: 27th June 2025, 11:07 pm
By Mandira Ganti, intern, Tech Communications
They say food is fuel, which turns out to be a perfect metaphor for understanding how electric vehicle batteries work — they’re built like lasagna.
Creating the perfect lasagna can be daunting, but it’s easy to eat (plus, it’s tasty!). Building a high-performance EV battery cell takes precision and planning, but understanding the layers inside is surprisingly simple. Both a battery cell and a lasagna contain a few essential layers, each with a distinct role.
Let’s dig in.
When you imagine an EV battery, you might picture the large, rectangular unit installed under the floor in most electric vehicles. That’s actually the battery pack — the structure that houses many individual batteries. Think of the battery pack like an industrial-sized oven holding multiple pans of lasagna. Our current EV battery packs contain at least 10 modules — think of these as the lasagna pans. In our current battery design, each module holds 24 battery cells, technically called pouch form factor cells — our 24 servings of lasagna. So even though it looks like one solid system, it’s really hundreds of coordinated components working together.
And it’s within those individual battery cells where some of today’s most cutting-edge innovations happen.
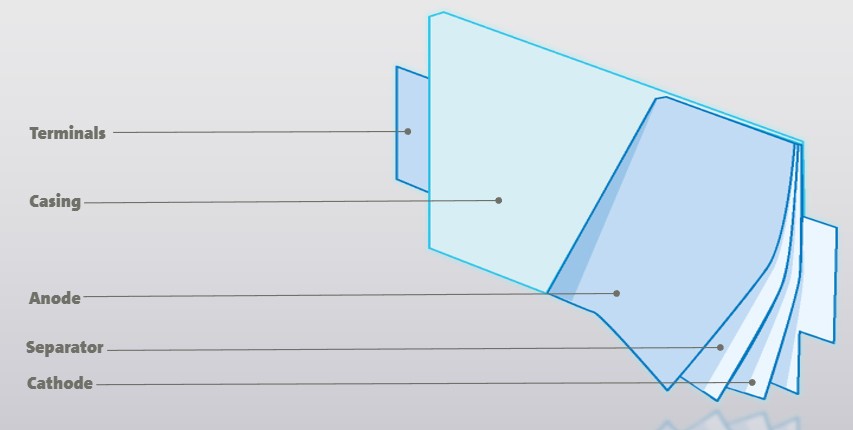
Now, let’s imagine opening a one-serving container of lasagna. That container is like the cell casing that protects one serving of battery power. Open the casing and you’ll find a stack of electrodes — think of this as your slice of lasagna (though in most GM EV batteries, the stack would be flipped on its side). Each battery cell has a pair of electrodes: a positively charged cathode and a negatively charged anode.
The electrodes are thin sheets of metal foil known as current collectors, aluminum for the cathode and copper for the anode, coated with different chemical “ingredients.” Think of the cathode as the rich, flavorful sauce layer, while the anode is like the creamy ricotta layer — both vital, but distinct parts of the recipe.
The key to good lasagna is keeping the ricotta and sauce from mixing into a gooey mess. In a lasagna, pasta sheets keep the layers distinct, while in a battery cell, a thin polymer separator keeps the cathode and anode materials apart. These three layers (cathode, separator, and anode) repeat multiple times to create a stack of roughly 20 layers. This structure is where the chemical reactions take place, storing the energy that powers an EV.

Without diving too deep just now, it’s important to understand some battery basics to really grasp the anatomy of a battery cell.
To create electric current, ions and electrons need to move between the electrodes via two paths.
Lithium ions travel from the anode, through the separator to the cathode inside the cell. The battery cell is filled with a gel-like substance called electrolyte which helps the ions pass through the separator. The electrolyte is like the water inside the cooked sheets of pasta that get layered into the lasagna — without the moisture from cooking, the pasta sheets would be too stiff, and the flavors (or in this case, ions) couldn’t flow between layers.
Meanwhile, electrons take an outside route, starting at the anode, traveling through the vehicle’s electrical systems, and returning to the cathode. The electrons enter and exit the cell through terminals, delivering electricity on the way to all the electrically-powered devices in the vehicle (the electric motor, the stereo, the air conditioning, etc.). This journey moves the power out of the battery into the car — the same way your fork delivers food from your plate to your mouth, then goes back for the next bite.
Electrons and ions repeat this journey from the cathode to the anode thousands of times per second, but it’s still all done with a few main parts:
- The cell casing
- Cathode sheets (aluminum current collector + chemical coating)
- Anode sheets (copper current collector + chemical coating)
- Separator sheets
- The cathode terminal
- The anode terminal
- The electrolyte
Advancements are constantly happening in EV battery tech, but these seven fundamental ingredients are always in the mix. When new EV battery technology is announced, it usually means engineers have fine-tuned one of these components, like a top chef adding a secret ingredient to improve their lasagna recipe. And since there are hundreds of battery cells in each electric vehicle, small changes can deliver significant leaps in performance, range, or cost.
So keep your eyes on the recipe. We’ll be back soon to explain how electrification technology works, using everyday concepts to make them easy to understand.
Article from GM.

Sign up for CleanTechnica’s Weekly Substack for Zach and Scott’s in-depth analyses and high level summaries, sign up for our daily newsletter, and follow us on Google News!
Whether you have solar power or not, please complete our latest solar power survey.
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Sign up for our daily newsletter for 15 new cleantech stories a day. Or sign up for our weekly one on top stories of the week if daily is too frequent.
CleanTechnica uses affiliate links. See our policy here.
CleanTechnica’s Comment Policy
This post has been syndicated from a third-party source. View the original article here.





